Phoenix Characteristics Vs. Others
Phoenix Pharma Labs’ drug candidates deliver potent pain relief with minimal side effects and no addiction risk.
Potent opioids with unique profiles that do not activate Mu or Kappa receptors—free from the serious side effects typically associated with both
| CHARACTERISTIC | MU OPIOIDS | KAPPA OPIOIDS | PPL-103 | PPL-138 |
|---|---|---|---|---|
| Significant Euphoria ("High" Feeling) ( CPP/SA ) |  |  |  |  |
| Significant Dysphoria ("Bad" Feeling) (CPA) |  |  |  |  |
| Lethal Respiratory Depression (Slowed Breathing) at Moderate Dose |  |  |  |  |
| Significant Constipation |  |  |  |  |
| Withdrawal Symptoms |  |  |  |  |
| Sustains Without Opiate Withdrawal |  |  |  |  |
| Drowsiness |  |  |  |  |
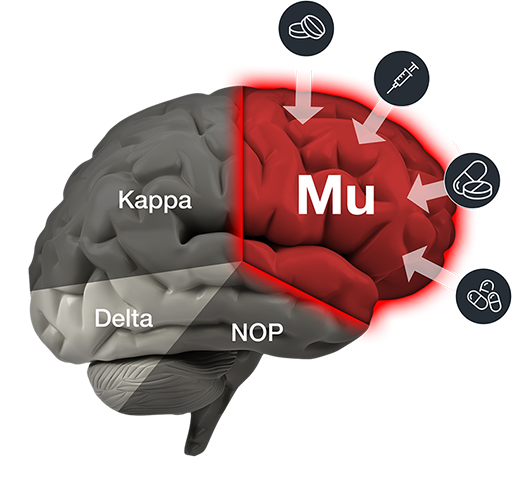
The Addiction Risk of Opioids
Mu produces potent pain relief, but it also produces a euphoric “high” – which leads to abuse and addiction.
PPL-138
Phoenix PharmaLabs has collaborated with scientists at the University of Bath (UK) in the development of a portfolio of 25 Nociceptin compounds including the drug that we now call PPL-138. This compound meets all of our criteria for a potent, safe, non-addicting analgesic for effective treatment of moderate to severe pain. It has demonstrated long duration of action, effective bioavailability in sublingual administration and no development of tolerance after four weeks of chronic administration. Since PPL-138 has such a long duration of action, it has excellent potential for development as a non-addictive treatment for chronic and acute pain.
PPL-138 Studies in Non-Human Primates:
- Robust analgesic potency – >100x the potency of morphine
- Long Duration of Action – >24 Hours
- Little or No Euphoria or Abuse-Liability – No self-administration preference [1]
- No Physical Dependence – No withdrawal symptoms
- No Respiration Depression – Low risk of lethal overdose
- No Tolerance – After 4 weeks of chronic administration
- No Constipation – At elevated dose levels
Technical Insights & Documentation
All technical documentation, findings, and peer-reviewed papers related to PPL-138 – available here in PDF format.

Research and Studies by
Leading Institutions
Studies of the drugs have been conducted by prominent scientists at leading institutions including Virginia Commonwealth University (VCU), the University of Michigan, Wake Forest University, SRI International, Torrey Pines Institute for Molecular Studies, ITR Canada, the University of Montreal, University of Bath and Florida Atlantic University (FAU).
Animal Health Applications of Our Drug Family
Recently our drug family has also attracted attention for Animal Health applications, primarily due to the lack of respiratory depression and GI tract side effects as well as the likelihood that the drugs would likely be unscheduled (or at most scheduled as Class IV).
Intellectual Property
Intellectual Property
Phoenix has a broad IP platform including over two dozen novel analogs. PPL-138 is in IND submission with Phase I trials planned for Q3, 2025. This provides the company with multiple grant opportunities as well as the flexibility to develop different products for different markets (e.g. acute and chronic pain, addiction therapies, PTSD and PTSD/AUD (alcohol use disorder) comorbidity, animal therapies, etc.).


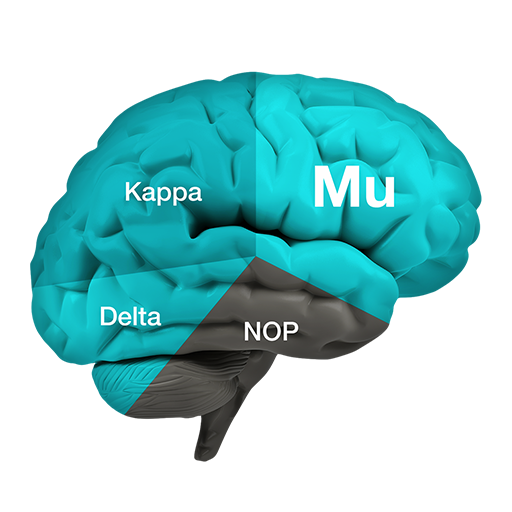
PPL-138 and other NOP Compounds
Phoenix Pharma Labs has exclusive license to 25 Nociceptin compounds called PPL-138. In December of 2014 a composition of matter patent covering all of the compounds was filed in the United States and Europe. It was issued in the US in May, 2018 and in Europe in November, 2020. It has been validated in the UK, France, Germany, Spain, Italy, Belgium, Netherlands, and Switzerland.
Formulations, processes and methods of synthesis as well as details concerning additional NCE variants in development are currently protected as trade secrets.
Phoenix Pharma Labs believes its unique compounds potentially offer pain sufferers, whether from acute or chronic conditions, new hope for potent pain relief without addiction or other serious opioid side effects. Additionally, our compounds offer significant potential for development as treatments for cocaine, methamphetamine, and other addictions. We intend to continue to develop new analogs within our portfolio of proprietary molecules.
PPL-138 Studies in Non-Human Primates:
- Robust analgesic potency >100x the potency of morphine
- Long duration of action >24 Hours
- No euphoric “high” / abuse & addiction
- No physical dependence or withdrawal
- No respiratory depression / death from overdose
- No Constipation – even at elevated doses.
PPL-138 binds to the NOP and mu receptors and then just partially stimulates those receptors. Both mu and NOP produce potent pain relief, but activation of the NOP receptor blocks the release of excessive dopamine, thus preventing the euphoria (and the resulting abuse & addiction).

