Where
Pharma Meets
Happiness
Phoenix is a pre-clinical pharmaceutical company driven by a mission to bring potent, safe pain therapies to market without risk of abuse and addiction.
Happy Smile
to your face
We are idea generators, goal
seekers, challenge-thirsty
professionals,


Powering Precision Tech

Providing a pathway to potent pain therapy without the risk of addiction and serious side effects
From Discovery to Impact
Translating scientific innovation into measurable patient outcomes.
Innovative Pharma Technologies
At Phoenix Pharma Labs, we leverage cutting-edge technologies to enhance the development, manufacturing, and distribution of our pharmaceutical products. Our commitment to innovation ensures that we consistently deliver safe, effective, and high-quality solutions to meet the evolving needs of the healthcare industry.

PPL-138
Our lead candidate for acute & chronic pain: PPL- 138, represents a novel new class of analgesics with robust preclinical validation of potent pain relief (100X potency of morphine), low risk of abuse/addiction, and no respiratory depression.

PPL-103
Our second drug candidate, PPL-103, shows strong potential for treating addiction and pain. It has proven to have a low risk of abuse/addiction and reduced opioid side effects, offering a safer alternative for pain management.

Phase I
We are advancing PPL-138 into Phase I human clinical trials by year end 2023.

Funding
More than $23 M has been raised to date from grants from the U.S. Army and NIH/NIDA, equity investment and investment in kind.
Innovative Pharma Technologies
At Phoenix Pharma Labs, we use advanced technologies to enhance our pharmaceutical products, ensuring safety, effectiveness, and high quality to meet the evolving needs of healthcare.
Phase I
We are advancing PPL-138 into Phase I human clinical trials by end of 2023, marking a major step in its development.
PPL-138
Our lead candidate for acute & chronic pain: PPL- 138, represents a novel new class of analgesics with robust preclinical validation of potent pain relief (100X potency of morphine), low risk of abuse/addiction, and no respiratory depression.
PPL-103
Our second drug candidate, PPL-103, shows strong potential for treating addiction and pain. It has proven to have a low risk of abuse/addiction and reduced opioid side effects, offering a safer alternative for pain management.
Funding
More than $23 M has been raised to date from grants from the U.S. Army and NIH/NIDA, equity investment and investment in kind.
Market
Both compounds have promising potential to capture a sizable portion of the $30B opioid therapeutics market.
The Problem
Opioids are the most widely prescribed drugs for treatment of moderate to severe pain. They are also the most powerful analgesics for treatment of acute and chronic pain. However, their use is plagued by serious side effects, including abuse and addiction, severe withdrawal, constipation, respiratory depression, and death from overdose.
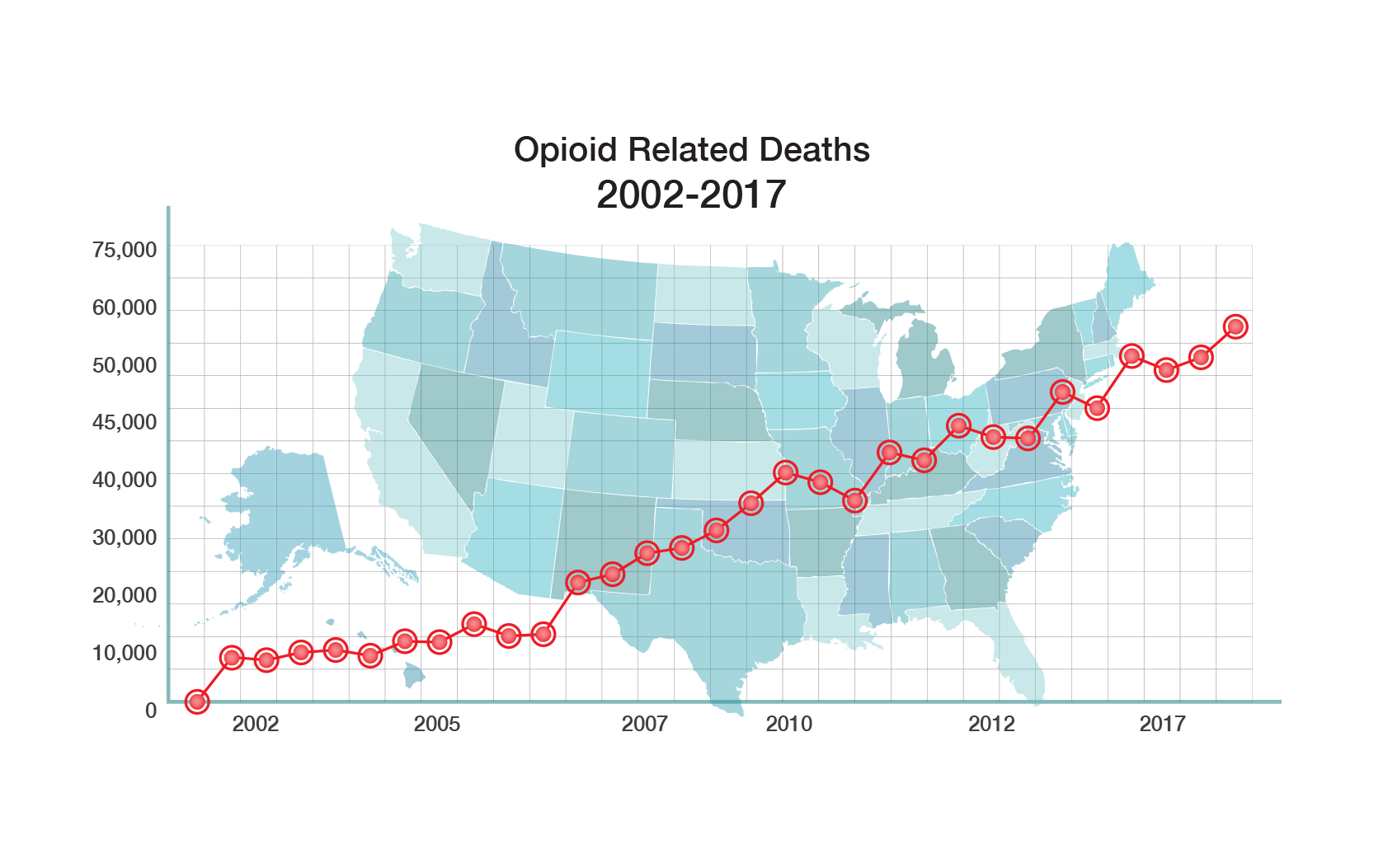
Next-Gen Pain Relief Without Addiction Risk
PPL has a portfolio of drugs under development with unique opioid receptor characteristics that do not produce the euphoric “high” that leads to abuse and addiction, while still treating moderate to severe acute and chronic pain without the dangerous side effects found in other opioids such as morphine, OxycodoneTM, HydrocodoneTM, etc.
We continue to seek complimentary business and investment relationships, out-licensing opportunities and/or co-development opportunities with both domestic and overseas partners as we advance our drug candidates through pre-clinical and clinical trials.
explore potential opportunities.
About Us
Phoenix PharmaLabs welcomes inquiries by organizations seeking a beneficial relationship. Please contact us to explore potential opportunities.
