Phoenix Characteristics Vs. Others
Phoenix Pharma Labs’ drug candidates deliver potent pain relief with minimal side effects and no addiction risk.
Potent opioids with unique profiles that do not activate Mu or Kappa receptors—free from the serious side effects typically associated with both
| CHARACTERISTIC | MU OPIOIDS | KAPPA OPIOIDS | PPL-103 | PPL-138 |
|---|---|---|---|---|
| Significant Euphoria ("High" Feeling) ( CPP/SA ) |  |  |  |  |
| Significant Dysphoria ("Bad" Feeling) (CPA) |  |  |  |  |
| Lethal Respiratory Depression (Slowed Breathing) at Moderate Dose |  |  |  |  |
| Significant Constipation |  |  |  |  |
| Withdrawal Symptoms |  |  |  |  |
| Sustains Without Opiate Withdrawal |  |  |  |  |
| Drowsiness |  |  |  |  |
The Addiction Risk of Opioids
Mu produces potent pain relief, but it also produces a euphoric “high” – which leads to abuse and addiction.
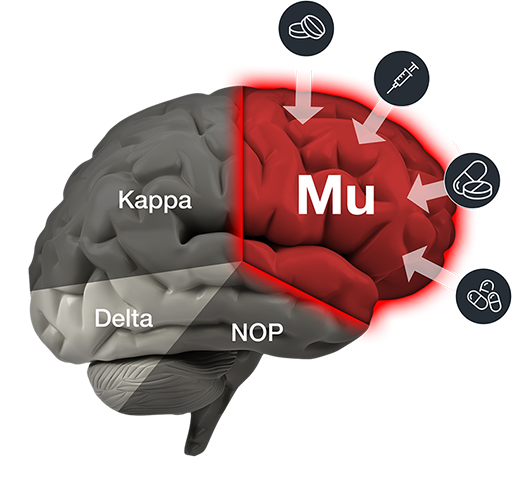
PPL-103
PPL-103 is unique because it binds strongly to three receptors: Mu, Kappa and Delta – and then just partially stimulates those receptors in a much more balanced manner. That partial stimulation derives analgesic benefit from all three receptors, but it is not sufficiently strong to produce the serious opioid side effects associated with any of the receptors. The reason that all of those other opioids strongly incentivize addiction is because they produce a euphoric “high”. Without that euphoria, opioid drugs would not be abused and consequently create addiction.
This profile results in first-ever opiate analgesics that appear to be non-addicting and free of significant dangerous side effects.
PPL-103 has clearly demonstrated in various animal studies that it does not produce euphoria (or dysphoria) and does not produce the other Mu opioid side effects like withdrawal, constipation and death from overdose.
PPL-103 Product Profile
- Robust analgesic potency – >10x the potency of morphine
- Little or No Euphoria or Abuse-Liability – >No self-administration preference [1]
- No Dysphoria – >No aversion – unlike kappa opioids
- No Physical Dependence – No withdrawal symptoms
- No Death from Overdose – At elevated dose levels
- No Constipation – At elevated dose levels
Technical Insights & Documentation
All technical documentation, findings, and peer-reviewed papers related to PPL-101 and PPL-103 – available here in PDF format.
PPL-138
Phoenix PharmaLabs has collaborated with scientists at the University of Bath (UK) in the development of a portfolio of 25 Nociceptin compounds including the drug that we now call PPL-138. This compound meets all of our criteria for a potent, safe, non-addicting analgesic for effective treatment of moderate to severe pain. It has demonstrated long duration of action, effective bioavailability in sublingual administration and no development of tolerance after four weeks of chronic administration. Since PPL-138 has such a long duration of action, it has excellent potential for development as a non-addictive treatment for chronic and acute pain.
PPL-138 Studies in Non-Human Primates:
- Robust analgesic potency – >100x the potency of morphine
- Long Duration of Action – >24 Hours
- Little or No Euphoria or Abuse-Liability – No self-administration preference [1]
- No Physical Dependence – No withdrawal symptoms
- No Respiration Depression – Low risk of lethal overdose
- No Tolerance – After 4 weeks of chronic administration
- No Constipation – At elevated dose levels

Technical Insights & Documentation
All technical documentation, findings, and peer-reviewed papers related to PPL-138 – available here in PDF format.

Research and Studies by
Leading Institutions
Studies of the drugs have been conducted by prominent scientists at leading institutions including Virginia Commonwealth University (VCU), the University of Michigan, Wake Forest University, SRI International, Torrey Pines Institute for Molecular Studies, ITR Canada, the University of Montreal, University of Bath and Florida Atlantic University (FAU).
