One compound,
three Indications
Providing a pathway to potent pain therapy without risk of addiction and serious side effects
Treat For Pain, Addiction, & PTSD
Effective painkiller without death from overdose


Relief Without
Risk

Empowering patients and providers with a safer choice in the fight against pain and addiction.
Pain Relief You Can Trust
Potent, safe, and non-addictive—because no one should have to choose between pain and risk.
Innovative Pharma Technologies
At Phoenix Pharma Labs, we leverage cutting-edge technologies to enhance the development, manufacturing, and distribution of our pharmaceutical products. Our commitment to innovation ensures that we consistently deliver safe, effective, and high-quality solutions to meet the evolving needs of the healthcare industry.

PPL-138
Our lead candidate for acute & chronic pain: PPL- 138, represents a novel new class of analgesics with robust preclinical validation of potent pain relief (100X potency of morphine), low risk of abuse/addiction, and no respiratory depression.

PPL-103
Our second drug candidate, PPL-103, shows strong potential for treating addiction and pain. It has proven to have a low risk of abuse/addiction and reduced opioid side effects, offering a safer alternative for pain management.

Phase I
We are advancing PPL-138 into Phase I human clinical trials by year end 2023.

Funding
More than $23 M has been raised to date from grants from the U.S. Army and NIH/NIDA, equity investment and investment in kind.
Many people in the world are in severe pain without access to adequate pain control because of fears of addiction liability.
Our Purpose
We are a purpose-driven drug development company, deeply committed to transforming lives through the creation of potent, safe, and non-addictive painkillers. Our focus extends to developing thoughtful, science-backed therapies for those navigating pain, addiction, and PTSD—conditions that impact billions of lives.
Moderate/ Severe Pain:
Moderate/ Severe Pain: PPL- 138 provides potent pain relief for acute & chronic pain, (100X potency of morphine), low risk of abuse/addiction, no respiratory depression, or constipation.
Addiction Treatment: PPL-138
Addiction Treatment: PPL-138 can block relapse in addicted subjects - making it a very promising treatment for addiction.
Treatment of PTSD
Treatment of PTSD: PPL-138 reduces stress and other PTSD symptoms - and also dramatically reduces associated alcohol consumption.
The Problem
Opioids are the most widely prescribed drugs for treatment of moderate to severe pain. However, their use is plagued by serious side effects, including abuse and addiction, severe withdrawal, constipation, respiratory depression, and death from overdose.
All of the leading opioids on the market today like morphine, Oxycodone, Hydrocodone, methadone, fentanyl, etc. – as well as heroin – bind to the Mu receptor in the brain and then aggressively stimulate that receptor. Mu produces potent pain relief, but it also produces a euphoric “high” – which leads to abuse and addiction.
More than XXX people die from overdose every year.
But that’s only part of the problem! Millions of people are addicted to opioids.
There are a lot of pain drugs out there, but NONE with this profile.
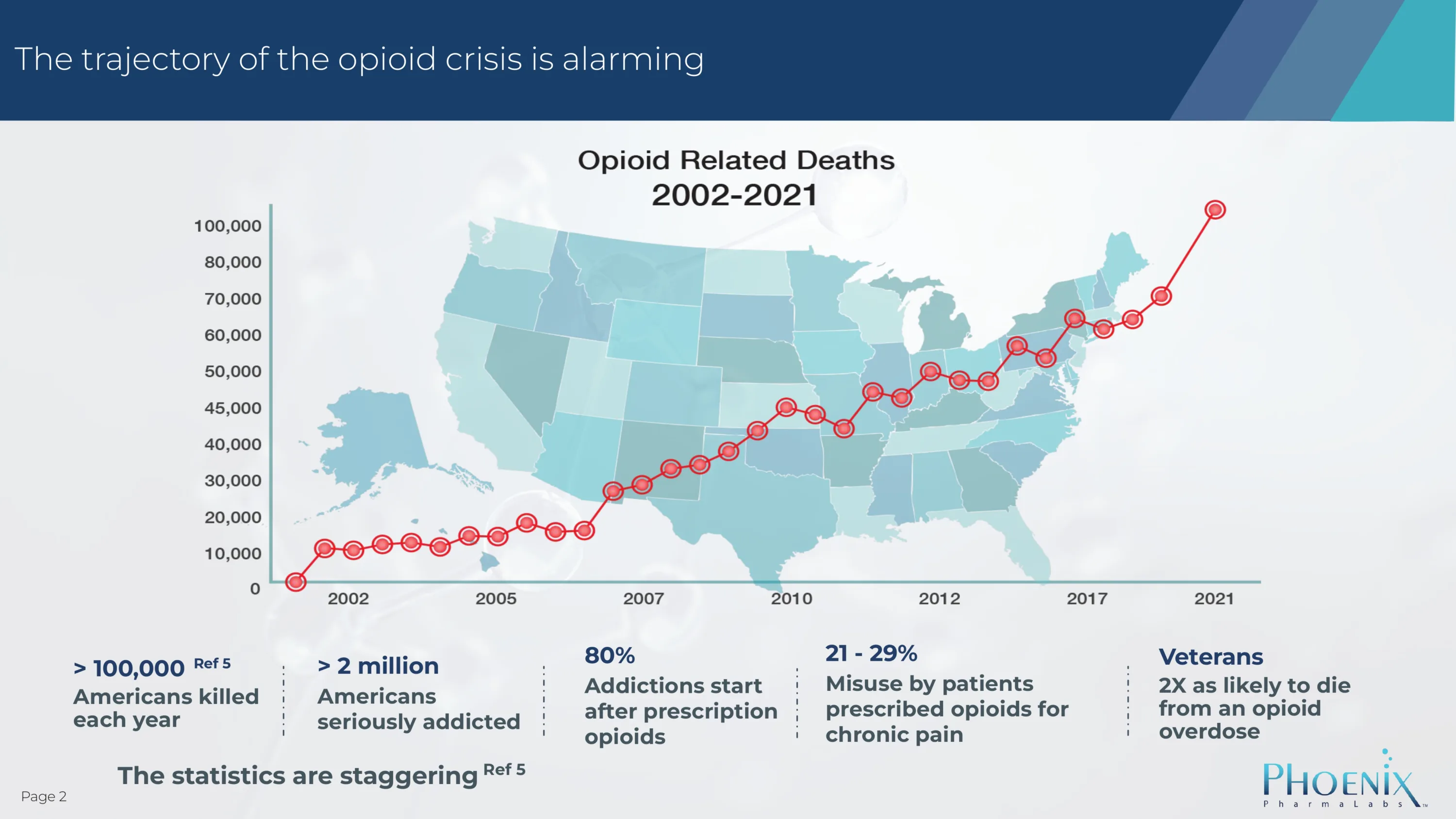
Millions of Americans are seriously addicted to opioids – and it is a devastating addiction. There is a huge unmet medical need to comprehensively address the abuse, addiction, and mortality associated with opioids.
Phoenix Pharma Labs has a portfolio of drugs under development with unique opioid receptor characteristics that do not produce the euphoric “high” that leads to abuse and addiction. These drugs treat moderate to severe acute and chronic pain without the dangerous side effects found in other opioids such as morphine, Oxycodone, Hydrocodone, etc.
We continue to seek complimentary business and investment relationships, out-licensing opportunities and/or co-development opportunities with both domestic and overseas partners as we advance our drug candidates through pre-clinical and clinical trials.
